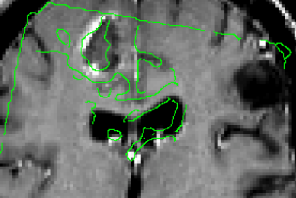
In some patients with refractory epilepsy that are candidates for surgery, intracranial EEG is recorded to precisely localize the epileptic focus. To record intracranial EEG, multiple depth electrodes are surgically implanted through holes in the skull, each with 8–15 equally spaced contacts. Current implantation planning consists of visual inspection of the patient’s MRI & CTA, visually searching for paths to the targets while avoiding vessels. This procedure is sub-optimal since estimation of the number of electrodes needed to sample a region and the precise location of each contact, which is paramount to accurately identify the focus and its extent, cannot be considered.
The goal of this project, conducted by Dr. Zelmann, one of my postdoctoral fellows, is to evaluate the clinical use of optimized depth electrode planning. We hypothesize that the use of our computed aided procedure will increase the accuracy and amount of information obtained during EEG intracranial investigation while constraining the trajectories to safe paths.
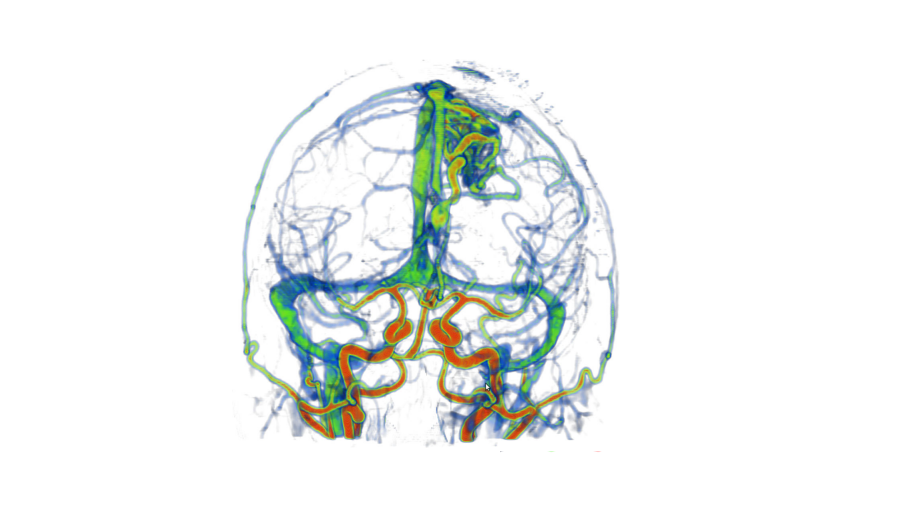
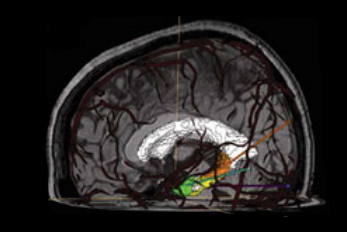
We have developed a procedure to optimize electrode location that would enable us to obtain complete coverage of a lesion or region of interest and surrounding neocortical grey matter, while minimizing the risk of approaching vessels and other critical structures. To this end, we automatically segment MRI, CT and CTA data; we model each electrode as a cylinder to assess risk; we estimate the contribution of individual contacts to record EEG; we compute an aggregated score for each electrode and a global score, obtaining the best cohort as the combined set of electrodes that remain at a safe distance.
To allow its clinical use during planning and surgical implantation, the procedure is integrated into an image-guided neuronavigation system developed in our research laboratory over the past decade, which is called IBIS (Interactive Brain Imaging System; Mercier et al., Int J CARS. 2011;6(4):507–522). IBIS allows planning and navigation based on preoperative medical image data. The best cohort and a list of possible electrodes per target (ordered in terms of risk and recorded EEG) is generated and displayed in IBIS and will be available during planning and in the OR. The surgeon can visually review trajectories, decide between one of the automatic trajectories and the manual one and, if necessary, re-plan a trajectory in almost real time.
Preliminary results [1] presented at an international workshop on clinical image-based procedures, suggest that automatic planning allows recording from a larger volume than manually planned trajectories (p<0.01) and from more temporal grey matter (p<0.001), while remaining further away from segmented vessels (p<0.01). We are currently evaluating the procedure in retrospective clinically acquired imaging data from 20 patients with electrodes implanted in MTL regions. We are comparing visual and automatic trajectories by estimating volume recording from the target volumes (in this case: amygdala and hippocampus) and neocortical temporal grey matter as well as distance to vessels and other critical structures.
Reference
[1] Zelmann, R., Beriault, S., Mok, K., Haegelen, C., Hall, J., Pike, G. B., Olivier, A & Collins, D. L. (2014). Automatic Optimization of Depth Electrode Trajectory Planning. In Clinical Image-Based Procedures. Translational Research in Medical Imaging (pp. 99–107). Springer International Publishing.
[2] R. Zelmann, S. Beriault, K. Mok, C. Haegelen, J. Hall, G.B. Pike, A. Olivier & D.L. Collins, “Improving Recorded Volume in Mesial Temporal Lobe by Optimizing Stereotactic Intracranial Electrode Implantation Planning” submitted to International Journal of Computer Assisted Radiology and Surgery (CARS-D-14–00251), Oct 9, 2014.
[3] Zelmann, R., Beriault, S., Mok, K., Haegelen, C., Hall, J., Pike, G. B., Olivier, A & Collins, D. L. (2014). Automatic Optimization of Depth Electrode Trajectory Planning. In Clinical Image-Based Procedures. Translational Research in Medical Imaging (pp. 99–107). Springer International Publishing.
[4] R. Zelman, D.L. Collins, “Automatic Optimization of Depth Electrode Trajectory Planning”, MICCAI 2013 Workshop on Clinical Image-based Procedures: Translational Research in Medical Imaging
[5] C. Haegelen, P. Perucca, C.-E. Châtillon, L. Andrade-Valença, R. Zelmann, J. Jacobs, D. L. Collins, F. Dubeau, A. Olivier and J. Gotman. “High-frequency oscillations, extent of surgical resection and surgical outcome in drug-resistant focal epilepsy”, Epilepsia 2013 May;54(5):848–57.
[6] Zelmann, R., S. Beriault, M. M. Marinho, K. Mok, J. A. Hall, N. Guizard, C. Haegelen, A. Olivier, G. B. Pike, and D. L. Collins. “Improving recorded volume in mesial temporal lobe by optimizing stereotactic intracranial electrode implantation planning.” International journal of computer assisted radiology and surgery (2015): 1–17.