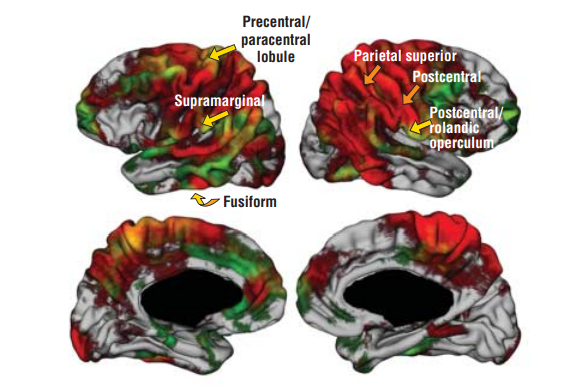
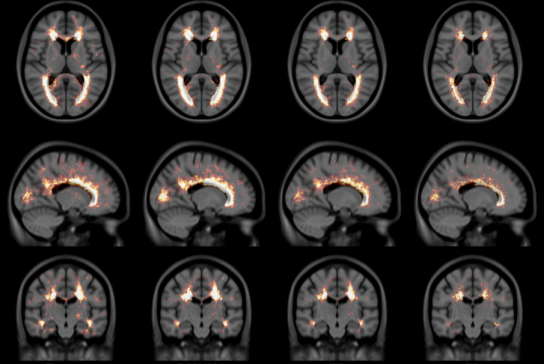
The NIST Lab has collaborated with the Utrecht group headed by Dr. H. Hulshoff Pol for the past number of years [1, 2, 3]. Her group has used my ANIMAL non-linear registration software with voxel based morphometry (VBM) for morphometric analysis of cerebral MRI of patients with schizophrenia. Recently they have provided evidence which suggests that brain structures reflect overlapping and segregating genetic liabilities for schizophrenia and BD. The overlapping smaller white matter volume and common areas of thinner and/or thicker cortex (thinner right orbitofrontal cortex, thinner right (and left) parahippocampus, and thicker temporoparietal and left superior motor cortices) suggest that both disorders share neurodevelopmental roots. They have also demonstrated excessive thinning of the cortex over time, most pronounced in the temporal and frontal areas, with progression across the span of the illness. This excessive thinning appears related to medication and outcome. Their findings were extended to reveal that cannabis use in patients with schizophrenia resulted in additional thinning in the left dorsolateral prefrontal cortex (DLPFC), left anterior cingulate cortex (ACC) and left occipital lobe as compared to those patients with schizophrenia that did not use cannabis after a 5-year follow-up.
In later papers, they have identified focal grey-matter differences in a number of regions when compared to age-matched controls [4]. They have also found focal white matter changes that suggest abnormal inter-hemispheric connectivity of anterior cortical and sub-cortical brain regions, reflecting decreased hemispheric specialisation in schizophrenia [5]. These results have been partially replicated in a twin study [6,7] and a long term follow-up study [8].
References
[1] Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, van Haren NE, Lepage C, Collins DL, Evans AC, Boomsma DI, Nolen W, Kahn RS. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry. 2012 Apr;69(4):349–59
[2] van Haren, Neeltje EM, Hugo G. Schnack, Wiepke Cahn, Martijn P. van den Heuvel, Claude Lepage, Louis Collins, Alan C. Evans, Hilleke E. Hulshoff Pol, and René S. Kahn. “Changes in cortical thickness during the course of illness in schizophrenia.” Archives of general psychiatry. 2011; 68(9): 871–880.
[3] M. Rais, N.E.M. van Haren, W Cahn, H.G. Schnack, C. Lepage, D.L. Collins, A. Evans, H. E. Hulshoff Poll and R.S. Kahn, “Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia,” European Neuropsychopharmacology, 2010 Dec; 20(12): 855–65.
[4] H. E. Hulshoff Pol, H. G. Schnack, R. C. Mandl, N. E. van Haren, H. Koning, D. L. Collins, A. C. Evans, and R. S. Kahn, “Focal gray matter density changes in schizophrenia,” Arch Gen Psychiatry. 2001; 58: 1118–25.
[5] H. E. Hulshoff Pol, H. G. Schnack, R. C. Mandl, W. Cahn, D. L. Collins, A. C. Evans, and R. S. Kahn, “Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity,” Neuroimage. 2004; 21: 27–35.
[6] H. Hulshoff Pol, H. G. Schnack, R. C. Mandl, R. G. H. Brans, N. E. van Haren, W. F. C. Baare, C. J. van Oel, D. L. Collins, A. C. Evans, and R. S. Kahn, “Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia: a voxel-based morphometry study,” presented at 10th Annual Meeting of the Organization for Human Brain Mapping, Budapest, Hungary, 2004.
[7] H. E. Hulshoff Pol, H. G. Schnack, R. C. Mandl, R. G. Brans, N. E. van Haren, W. F. Baare, C. J. van Oel, D. L. Collins, A. C. Evans, and R. S. Kahn, Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry, Neuroimage. 2006; 31(2): 482–8.
[8] N. E. van Haren, H. E. Hulshoff Pol, H. G. Schnack, W. Cahn, R. C. Mandl, D. L. Collins, A. C. Evans, and R. S. Kahn, “Focal Gray Matter Changes in Schizophrenia across the Course of the Illness: A 5-Year Follow-Up Study,” Neuropsychopharmacology. 2007; 32: 2057–66.